Position Your Brand at the Heart of the TIL Revolution in 2026
As the only summit laser-focused on the clinical, manufacturing, and regulatory advancement of TIL therapies, this annual event offered unmatched access to a highly engaged, technically sophisticated audience. With a refined track structure meaning we’ve got attendance from CMC, R&D and clinical leads, the 7th TIL Therapies Summit was your most targeted opportunity to grow brand visibility, generate leads, and build lasting partnerships in this fast-evolving space.
Our Audience are Looking for Support With:
CDMOs:
Provide GMP-compliant manufacturing, expansion, and quality control services for TIL products.
Gene Engineering: Offer tools, services, and expertise to genetically modify TILs, often through simplified, kit-based technologies.
Immune Monitoring: Deliver assays and sequencing technologies (e.g., flow cytometry, TCR sequencing, spatial biology) to understand TIL composition and mechanism of action.
Vector Production: Supply GMP-grade viral or non-viral vectors used to deliver gene-editing technologies into TILs.
Blood Banks: Provide large quantities of feeder cells from healthy donors, essential for TIL expansion in both research and clinical-scale manufacturing.
Why Partner in 2026?
Brand Exposure
Position your brand at the center of the TIL revolution. Whether you offer bioreactors, feeder cell alternatives, sequencing platforms, or automation tools, this summit connects you with companies actively seeking your solutions.
Thought Leadership
Showcase your innovations in TIL manufacturing, gene editing, immune monitoring, and neoantigen discovery. Lead conversations that shape the future of TIL therapy development.
Networking Opportunities
Meet decision-makers from biotech, pharma, and academia who are looking for strategic partners to overcome key challenges in TIL scale-up, QC, and regulatory compliance.
Harness Market Intelligence
Gain firsthand insights into the evolving needs of TIL developers - from computational tools and IL-2 replacements to feeder cell QC and access to rare cell types.
Who Did Our 2025 Partners Meet?
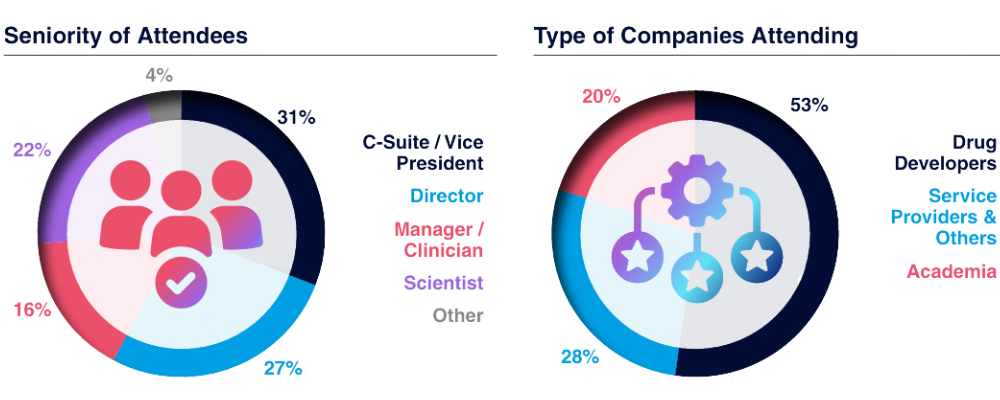
*Statistcs Taken from the 6th TIL Therapies Summit 2024
Our 2025 Partners:
Expertise Partners



Program Partner

Innovation Partner

Exhibition Partner

Event Partner

View the 2026 Cell Therapy Partnership Prospectus